New benzylidene oxazolone derived polymeric photoswitches for light-induced tunable thermoresponsive behaviors†
Abstract
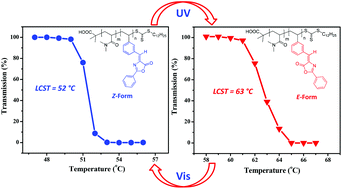
A series of thermoresponsive polymeric photoswitches containing benzylidene oxazolone moieties were successfully prepared. 3-Vinylbenzaldehyde (VBA) and dimethylacrylamide (DMA) were copolymerized by reversible addition–fragmentation chain transfer (RAFT) polymerization to obtain statistical copolymers, poly(3-vinylbenzaldehyde-co-dimethylacrylamide) (PVBA-co-PDMA). The resulting PVBA-co-PDMA copolymers were reacted with hippuric acid to yield the target copolymers, poly(3-vinylbenzylidene oxazolones-co-dimethylacrylamide) (PVBO-co-PDMA). Successful synthesis of the desired copolymers was confirmed by 1H-NMR spectroscopy and gel permeation chromatography (GPC). UV-Vis absorption measurements were employed to study the efficiency of photoisomerization of the benzylidene oxazolone moieties upon irradiation with UV and visible light (365 nm and 650 nm) for various time intervals. Temperature-dependent optical transmission studies (turbidimetry) were also carried out on the aqueous solutions to investigate how the lower critical solution temperature (LCST) values were influenced by photoirradiation. The profiles of optical transmittance suggested that the LCST of the E isomer was higher than that of the Z isomer. The control of LCST by photoirradiation was observed to be repeatable over multiple cycles, demonstrating that photoisomerization is reversible. The LCST values measured by dynamic light scattering (DLS) correlated well with those determined by turbidimetry.

 Please wait while we load your content...
Please wait while we load your content...