Synthesis and chiral recognition ability of helical polyacetylenes bearing helicene pendants†
Abstract
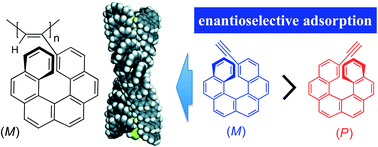
Novel polyacetylenes bearing an optically active or racemic [6]helicene unit as the pendant groups directly bonded to the main-chain (poly-1s) were prepared by the polymerisation of the corresponding acetylenes (1-rac, 1-P and 1-M) using a rhodium catalyst. The optically active polyacetylenes (poly-1-P and poly-1-M) formed a preferred-handed helical conformation biased by the optically active helicene pendants, resulting in the induced circular dichroism (ICD) in their π-conjugated polymer backbone regions. The optically active helical polymers, when employed as an enantioselective adsorbent, showed a high chiral recognition ability towards racemates, such as the monomeric [6]helicene and 1,1′-binaphthyl analogues, and enantioselectively adsorbed one of the enantiomers.

 Please wait while we load your content...
Please wait while we load your content...