Controlled polymerization of histidine and synthesis of well-defined stimuli responsive polymers. Elucidation of the structure–aggregation relationship of this highly multifunctional material†
Abstract
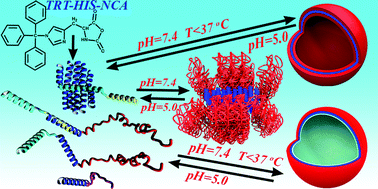
We present the synthesis of the novel monomer Nim-trityl-protected N-carboxy anhydride of L-histidine (Trt-HIS-NCA) for the synthesis of poly(L-histidine) (PHIS). Kinetic studies of the ring opening polymerization of Trt-HIS-NCA followed first order kinetics, indicating that the polymerization is “living”. The high purity of the synthesized monomer along with the use of high vacuum techniques resulted in the controlled polymerization of histidine in a variety of macromolecular architectures exhibiting high degrees of molecular and compositional homogeneity. The conformation of poly(L-histidine) (PHIS) was studied at different pH values and temperatures by circular dichroism, revealing that it adopts a random coil conformation at low pH and temperatures, a β-sheet conformation at higher pH, and probably a broken β-sheet conformation at higher temperatures. We found that the pKa of the PHIS homopolymer depends on the molecular weight. Addition of hydrophobic amino acids randomly distributed along the PHIS chain hinders the organization of PHIS, resulting in the formation of the random coil conformation even at higher pH. The influence of either leucine (LEU) or γ-benzyl-L-glutamate (BLG) randomly distributed along the PHIS chain on the pKa and degree of protonation in the terpolymers revealed that although the pKa is lower, the protonation of PHIS increases at lower pH values, while it is lower at a higher pH as compared to that obtained for PHIS. The aggregates of PEO-b-P(HIS-co-PLEU(BLG)) in water were found to swell more by decreasing the pH and increasing the hydrophobic amino acids, and eventually become disrupted. Surprisingly, at pH = 7.4, the increase in temperature leads to lower aggregation of the PEO-b-PHIS due to the transition of the secondary structure. The results indicate that it is possible to fine-tune the protonation of PHIS as a function of pH and temperature, and thus to control the conditions where the aggregates will be disrupted, a prerequisite for drug and gene delivery applications.

 Please wait while we load your content...
Please wait while we load your content...