Synthesis of poly(ethylene glycol)-b-poly(N-(2-hydroxypropyl) methacrylamide) block copolymers with well-defined structures and their influence on in vivo circulation and biodistribution†
Abstract
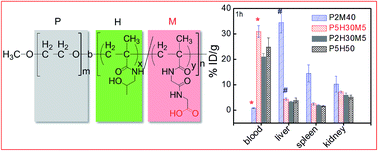
Well-defined water-soluble block copolymers poly(ethylene glycol)-b-poly(N-(2-hydroxypropyl) methacrylamide-co-N-methacryloylglycylglycine) (PEG-b-P(HPMA-co-MAGG)) with different compositions and narrow polydispersity were synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization. The in vivo blood clearance and biodistribution of copolymers with different compositions were studied in normal BALB/c mice. The results showed that the electronegative copolymers were rapidly eliminated from blood and tended to accumulate in the liver and spleen. However, the copolymers with neutral or a small amount of negative charges showed a prolonged circulation time and low non-specific organ uptake. Combined with the quantitative analysis of in vitro hepatocyte uptake, we conclude that this was due to the balance between (i) the electrostatic repulsion between the copolymer and the cell membrane and (ii) the tendency of macrophage-like cells to capture the negative charged copolymers. This work also revealed the significant roles of the PEG chain length, negative charge and molecular weight for the copolymers as anticancer drug carriers with prolonged circulation time and optimal biodistribution.

 Please wait while we load your content...
Please wait while we load your content...