Chain-growth cationic polymerization of 2-halogenated thiophenes promoted by Brønsted acids†
Abstract
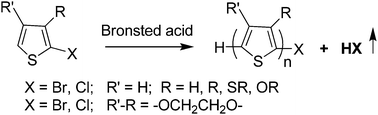
Brønsted acids are found to be effective and generally applicable catalysts for inducing the cationic chain-growth polymerization of a wide variety of 2-halogenated-3-substituted-thiophenes with hydrogen, alkyl, alkylthio, alkoxy, and dialkoxy substituent groups. The effectiveness of the cationic polymerization depends on the acid strength and the electron density of the 2-halothiophene monomer. For the most electron-rich monomer, like EDOT, the polymerization can be initiated essentially with any acid that is stronger than acetic acid. While for the electron-poor 2-bromothiophene, it only works with the strongest acid, e.g., trifluoromethanesulfonic acid. Using this new polymerization method, highly solution-processable and conductive poly(alkylthiothiophene)s with a conductivity greater than 180 S cm−1 can be conveniently prepared. Control experiments indicate that Lewis acid like BF3 and SnCl4 (0.5 equiv.) can also induce the polymerization of 3-alkylthio-2-bromothiophenes in a similar fashion, but their polymerization failed totally in the presence of a trace amount of acid scavenger (e.g., <0.1 equiv. NEt3). On the other hand, similar polymerizations with Brønsted acid did not show any noticeable adverse effect under the same condition. The polymerization with Brønsted acid may involve the coupling between the monomer (as the nucleophile) and its protonated form (as the electrophile), followed by the elimination of HBr (or HCl) to convert the unstable nonconjugated dimeric intermediate 11 (

 Please wait while we load your content...
Please wait while we load your content...