Design of coordination polymers with 4′-substituted functionalized terpyridyls in the backbone and pendent cyclopentadienyliron moieties
Abstract
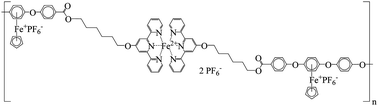
The reaction of dichloro-terminated organoiron complex [Fe(1,4-C6H4Cl2)Cp]+PF6− (3) with 4-hydroxybenzoic acid resulted in the formation of dicarboxylic acid organoiron complex 4. 4′-Hexyl alcohol-2,2′:6′,2′′-terpyridine (hextpy) 2 was reacted with dicarboxylic acid organoiron complex 4 or the similar monoacid organoiron complex 5 to afford two novel organoiron complexes containing one or two terminal terpyridine moieties (complexes 6 and 7, respectively). Molecular dynamics simulations of 7 revealed that the terminal terpyridine units readily interact with one another. The metal-containing complexes [M(hextpy)2](PF6)2 {M = Fe (8), Ni (9)} were prepared from the reaction of FeCl2·4H2O or Ni(CH3COO)2·4H2O with hextpy 2. The products were fully characterized by IR, UV-visible, and NMR spectroscopies, as well as elemental analysis. The reaction of monocarboxylic acid organoiron complex 5 with bis(hextpy) complexes 8 and 9 afforded the novel chloro-terminated monomers 10 and 11 containing two different metals. The reaction of monomer 7 with iron(II) chloride or nickel(II) acetate in methanol produced iron(II)- or nickel(II)-containing polymers 12 (M = Fe) and 13 (M = Ni), respectively. The monomers containing multimetal centers were polymerized through aromatic substitution reactions with bisphenol A or hydroquinone to afford polymers 14 and 15, respectively. The thermal properties of the polymers were also investigated, providing glass transition temperatures of approximately −25 °C for the iron-chelated polymers and a stepwise degradation of the polymers, beginning with the decoordination of the pendent cationic iron moieties and ending with the loss of the bonding interaction between the iron center and the nitrogen atoms of the chelated terpyridine groups.

 Please wait while we load your content...
Please wait while we load your content...