Light-responsive fluorescent cross-linked polymeric micelles based on a salicylidene Schiff base pendant-functionalized block copolymer
Abstract
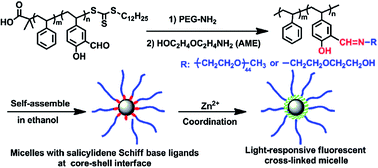
A well-defined block copolymer polystyrene-block-poly(2-hydroxy-5-vinylbenzaldehyde) (PS-b-PHVB) was synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization of HVB using a trithiocarbonate terminated polystyrene macro-chain transfer agent. Sequential grafting of monoamine-terminated PEG (PEG–NH2) and 2-(2-aminoethoxy)ethanol (AME) onto the HVB blocks of PS-b-PHVB via aldehyde–amine condensation afforded an amphiphilic block copolymer PS-b-(PHVB-g-PEG-and-AME) bearing a pendant salicylidene Schiff base with a precise structure. PS-b-(PHVB-g-PEG-and-AME) could self-assemble in ethanol into micelles with salicylidene Schiff base groups at the core–shell interface. Addition of Zn2+ ions into the resulting micellar solution not only endowed the micelles with fluorescent features but also enabled the resulting luminescent micelles to be stabilized through ionic cross-linking in consequence of the salicylaldimine–Zn2+ complexation. The obtained fluorescent cross-linked micelle was “light-controllable” in terms of its fluorescence emission and cross-linking structure due to the reversibility of the salicylaldimine–Zn2+ coordinative bond in response to light stimuli. The reversible fluorescence decrease and recovery could be achieved periodically upon alternative 254 nm UV irradiation and maintaining in the dark, and the micelles could be de-stabilized by UV irradiation-induced de-cross-linking. The capture of guest molecules and the light-triggered release of the guests for this Zn2+ coordinated micelle were also demonstrated using the coumarin-153 dye as a model guest molecule.

 Please wait while we load your content...
Please wait while we load your content...