High-speed organocatalytic polymerization of a renewable methylene butyrolactone by a phosphazene superbase
Abstract
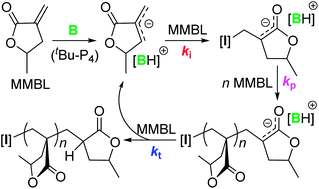
The organic phosphazene superbase, 1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)phosphoranylid-enamino]-2λ5,4λ5-catenadi(phosphazene) (t-Bu-P4), is found to directly initiate high-speed polymerization of the biomass-derived renewable γ-methyl-α-methylene-γ-butyrolactone (MMBL), in contrast to other polymerization systems using t-Bu-P4 which typically require addition of an organic acid or a nucleophile as a co-initiating component. This MMBL polymerization by t-Bu-P4 alone is extremely rapid; even with a low t-Bu-P4 loading of 0.1 mol% or 0.02 mol%, quantitative monomer conversion is achieved in 20 s or 1 min, respectively, affording medium to high molecular weight PMMBL bioplastics in a catalytic fashion. The combined experimental and theoretical/computational studies have yielded mechanisms of chain initiation through abstraction of a proton from a monomer by t-Bu-P4, essentially barrier-less chain propagation through rapid conjugate addition of the enolate anion stabilized by the nano-size cation [t-Bu-P4H]+ to the monomer, and chain termination through chain transfer to the monomer which generates a saturated termination chain end and the [t-Bu-P4H]+-stabilized anionic active species that starts a new chain.
- This article is part of the themed collection: Sustainable polymers: replacing polymers derived from fossil fuels

 Please wait while we load your content...
Please wait while we load your content...