Enzyme-catalyzed ring-opening polymerization of ε-caprolactone using alkyne functionalized initiators†
Abstract
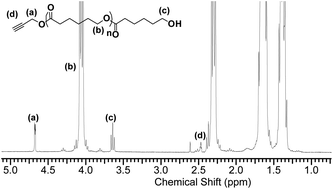
Well-defined poly(caprolactone)s were generated using alkyne-based initiating systems catalyzed by Candida antarctica Lipase B (CALB). Propargyl alcohol and 4-dibenzocyclooctynol (DIBO) were shown to efficiently initiate the polymerizations under metal free conditions and yielded 4 to 24 KDa polymers with relatively narrow molecular mass distribution. The alkyne bonds in the strained and unstrained initiating systems survived the polymerization conditions and the purification process. The resulting bonds can be sequentially functionalized post-polymerization using metal free and copper catalyzed click chemistry conditions.

 Please wait while we load your content...
Please wait while we load your content...