The effect of pendant benzene-1,3,5-tricarboxamides in the middle block of ABA triblock copolymers: synthesis and mechanical properties†
Abstract
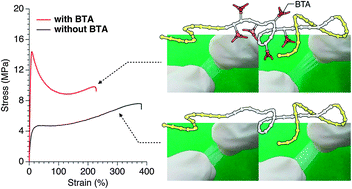
The synthesis and mechanical properties of ABA triblock copolymers containing benzene-1,3,5-tricarboxamide (BTA) moieties in the middle block are described. The triblock architecture was achieved by sequential polymerization of different monomers by atom-transfer radical polymerization (ATRP). The ABA triblock copolymer has a soft–hard–soft block sequence, in which the “A” block consists of soft poly(methyl acrylate), while the “B” block is a random copolymer of isobornyl methacrylate with 20 mol% of propargyl methacrylate partially functionalized with peripheral BTA groups. The pendent BTAs self-assemble into helical aggregates through lateral hydrogen-bond formation. Thermal and mechanical analyses indicated that the Young's modulus is enhanced by the BTAs. AFM images revealed that BTA self-assembly has dramatic influence on the nanoscopic ordered structure. The morphology of the triblock copolymer without BTAs consisted of hard, isolated domains embedded in a soft matrix. The copolymer containing BTAs appears as a continuous, disorganized morphology with nanoscopic domain sizes. This morphological difference presumably influences the Young's modulus. Ductility (i.e., necking) was only observed in the polymer containing BTAs. From these investigations, we conclude that introducing BTA in the hard-midblock results in intermolecular physical crosslinks, and the morphological characteristics translate to improved strength as reflected by the modulus.

 Please wait while we load your content...
Please wait while we load your content...