Drug release of pH-sensitive poly(l-aspartate)-b-poly(ethylene glycol) micelles with POSS cores†
Abstract
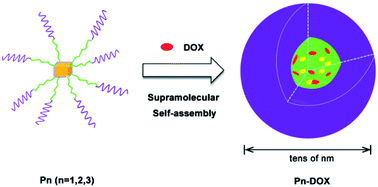
Amphiphilic poly(L-aspartate)-b-poly(ethylene glycol) block copolymers were synthesized and immobilized on polyhedral oligomeric silsesquioxanes (POSS) to obtain star shaped copolymers. 1-(3-Aminopropyl) imidazole was conjugated to the pendant groups of poly(L-aspartate) segments to fabricate pH-sensitive micelles. The anticancer drug doxorubicin (DOX) was trapped in the micelles to investigate the effects of side groups on the drug release behaviors. The mean diameters of DOX loaded micelles were around 50–60 nm, which were much smaller than those of blank micelles (100–200 nm). Both the drug loading content and encapsulation efficiency of the micelles decreased with the sequence of the side group substitution of carboxyl, benzyl and imidazole. The release of DOX loaded micelles with imidazole groups was pH-dependent, and more than 90% of the loaded DOX could be released within 48 hours in a weak acidic medium (pH 5.0). The flow cytometry and confocal laser scanning microscopy results revealed that the pH-sensitive micelles exhibited better anticancer activity.

 Please wait while we load your content...
Please wait while we load your content...