Reversible photocontrol of self-assembled peptide hydrogel viscoelasticity†
Abstract
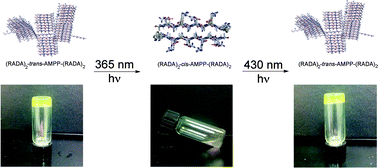
Peptide hydrogels are promising biomaterials for applications ranging from drug delivery to tissue engineering. Peptide hydrogels that change their physical properties in response to an exogenous stimulus are advantageous as biomaterials that can be temporally controlled. Herein, we report the use of an azobenzene turn mimetic, [3-(3-aminomethylphenylazo)phenyl]acetic acid (AMPP), to engineer a light-responsive β-hairpin into the center of a hydrogel-forming peptide. In the trans state, AMPP exists in a β-arc conformation, and the peptide forms a rigid self-supporting gel. The peptide hydrogel rigidity is reduced upon trans–cis azobenzene isomerization, which promotes formation of putative β-hairpin assemblies. This process is reversible in that hydrogel viscoelasticity is restored upon reverse cis–trans photoisomerization. TEM imaging and spectroscopic data reveal that the loss in rigidity is a result of disruption of the well-ordered macromolecular structure and not due to disassembly of the constituent self-assembled β-sheet fibrils. These findings provide insight into the effect of β-arc and β-hairpin turns on the emergent properties of self-assembled peptide hydrogels and provide a basis for temporal control of hydrogel rigidity using near-UV light.

 Please wait while we load your content...
Please wait while we load your content...