In vitro sensing of Cu+ through a green fluorescence rise of pyranine†
Abstract
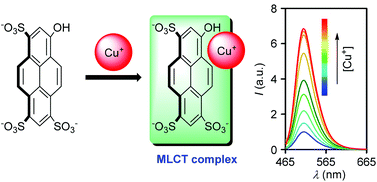
Pyranine as a new class of fluorescent chemosensor for the Cu+ ion is reported. The probe is capable of discriminating ranges of cations from the Cu+ ion, even in competing environment. The dye displayed a rapid fluorescence response (t1/2 = 1.66 min) towards the Cu+ ion, and the micromolar detection limit enabled the detection of the ion in environmental samples. The observed stoichiometry of complexation between pyranine and Cu+ was 2 : 1. Interestingly, the sensing characteristic was specific to only neutral pH. A metal-to-ligand charge-transfer (MLCT)-based mechanism of sensing was proposed based on electron spin resonance (EPR), Raman spectroscopic and cyclic voltammetric studies.

 Please wait while we load your content...
Please wait while we load your content...