Abstract
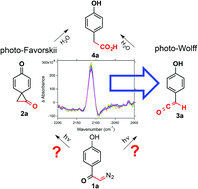
α-Diazo arylketones are well-known substrates for Wolff rearrangement to phenylacetic acids through a ketene intermediate by either thermal or photochemical activation. Likewise, α-substituted p-hydroxyphenacyl (pHP) esters are substrates for photo-Favorskii rearrangements to phenylacetic acids by a different pathway that purportedly involves a cyclopropanone intermediate. In this paper, we show that the photolysis of a series of α-diazo-p-hydroxyacetophenones and p-hydroxyphenacyl (pHP) α-esters both generate the identical rearranged phenylacetates as major products. Since α-diazo-p-hydroxyacetophenone (1a, pHP N2) contains all the necessary functionalities for either Wolff or Favorskii rearrangement, we were prompted to probe this intriguing mechanistic dichotomy under conditions favorable to the photo-Favorskii rearrangement, i.e., photolysis in hydroxylic media. An investigation of the mechanism for conversion of 1a to p-hydroxyphenyl acetic acid (4a) using time-resolved infrared (TRIR) spectroscopy clearly demonstrates the formation of a ketene intermediate that is subsequently trapped by solvent or nucleophiles. The photoreaction of 1a is quenched by oxygen and sensitized by triplet sensitizers and the quantum yields for 1a–c range from 0.19 to a robust 0.25. The lifetime of the triplet, determined by Stern–Volmer quenching, is 31 ns with a rate for appearance of 4a of k = 7.1 × 106 s−1 in aq. acetonitrile (1 : 1 v : v). These studies establish that the primary rearrangement pathway for 1a involves ketene formation in accordance with the photo-Wolff rearrangement. Furthermore we have also demonstrated the synthetic utility of 1a as an esterification and etherification reagent with a variety of substituted α-diazo-p-hydroxyacetophenones, using them as synthons for efficiently coupling it to acids and phenols to produce pHP protect substrates.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...