Manipulating γ-cyclodextrin-mediated photocyclodimerization of anthracenecarboxylate by wavelength, temperature, solvent and host†
Abstract
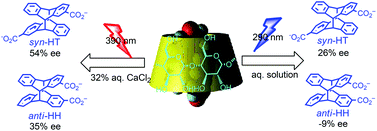
Wavelength effects on the enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate (AC) mediated by native and modified γ-cyclodextrins (CDs) were examined in different solvents at varying temperatures to manipulate the photochirogenic outcomes beyond the thermodynamically determined re/si-enantiotopic face selectivity upon 2 : 1 complexation of AC with CD in the ground state. Indeed, the stereochemical outcomes, i.e. syn/anti, head-to-tail/head-to-head (HT/HH) and in particular enantiomer ratios, were critical functions of the irradiation wavelength, irrespective of the CD host employed. Furthermore, the wavelength effects observed strongly depended on the host structure, reaction temperature and solvent employed, for which altered stacking geometry of the complexed AC pair is thought to be responsible. By optimizing the irradiation wavelength, chiral host, temperature and solvent, an enantiomeric excess of up to 54 and −37% were achieved for chiral syn-HT and anti-HH dimers.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...