Abstract
A photochromic diarylethene having 2-methyl-4-pyridyl groups formed chiral cocrystals with (R)- or (S)-1,1′-bi-2-naphthol (BINOL). X-ray crystal structure analysis revealed that the diarylethene forms O–H⋯N type hydrogen bonds with BINOL and the central hexatriene moiety is fixed in P- or M-helix conformation in the cocrystals. The diarylethene molecules underwent reversible cyclization reactions in the single-component crystal as well as in the cocrystals upon alternate irradiation with ultraviolet (UV) and visible light. In the chiral cocrystals, a highly enantioselective photocyclization reaction took place owing to the conformational confinement.
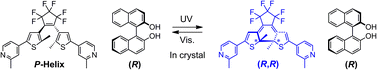
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...