Insights into the Pummerer synthesis of oxazolines†
Abstract
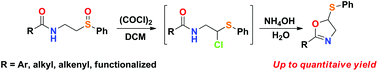
A rapid and simple method to access unnatural 2-substituted 5-thio oxazolines has been developed. This methodology is based on a Pummerer reaction followed by an intramolecular nucleophilic substitution, which changes the paradigm for the normal use of a base in Pummerer chemistry. We also provide a useful two-step method for the synthesis of the starting material and a mechanistic proposal based on experimental observations, which contests the previously proposed reaction pathway. The reaction proved to be general, and different substituents, such as alkyl, aryl, alkenyl and functionalized groups, can be used without a significant decrease in efficiency.

 Please wait while we load your content...
Please wait while we load your content...