Asymmetric syntheses of three-membered heterocycles using chiral amide-based ammonium ylides†
Abstract
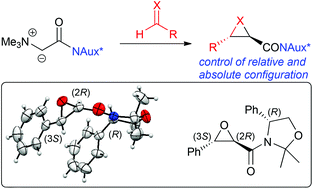
The use of carbonyl-stabilised ammonium ylides to access chiral glycidic amides and the corresponding aziridines has so far been limited to racemic trans-selective protocols. We herein report the development of an asymmetric approach to access such compounds with high levels of stereoselectivity using easily accessible chiral auxiliary-based ammonium ylides. The use of phenylglycinol as the chiral auxiliary was found to be superior to Evans or pseudoephedrine-based auxiliaries resulting in good to excellent stereoselectivities in both, epoxidation and aziridination reactions.
 Please wait while we load your content...
Please wait while we load your content...