Synthesis of unsaturated phosphatidylinositol 4-phosphates and the effects of substrate unsaturation on SopB phosphatase activity†
Abstract
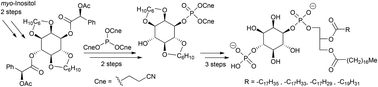
In this paper evidence is presented that the fatty acid component of an inositide substrate affects the kinetic parameters of the lipid phosphatase Salmonella Outer Protein B (SopB). A succinct route was used to prepare the naturally occurring enantiomer of phosphatidylinositol 4-phosphate (PI-4-P) with saturated, as well as singly, triply and quadruply unsaturated, fatty acid esters, in four stages: (1) The enantiomers of 2,3:5,6-O-dicyclohexylidene-myo-inositol were resolved by crystallisation of their di(acetylmandelate) diastereoisomers. (2) The resulting diol was phosphorylated regio-selectively exclusively on the 1-O using the new reagent tri(2-cyanoethyl)phosphite. (3) With the 4-OH still unprotected, the glyceride was coupled using phosphate tri-ester methodology. (4) A final phosphorylation of the 4-O, followed by global deprotection under basic then acidic conditions, provided PI-4-P bearing a range of sn-1-stearoyl, sn-2-stearoyl, -oleoyl, -γ-linolenoyl and arachidonoyl, glycerides. Enzymological studies showed that the introduction of cis-unsaturated bonds has a measurable influence on the activity (relative Vmax) of SopB. Mono-unsaturated PI-4-P exhibited a five-fold higher activity, with a two-fold higher KM, over the saturated substrate, when presented in DOPC vesicles. Poly-unsaturated PI-4-P showed little further change with respect to the singly unsaturated species. This result, coupled with our previous report that saturated PI-4-P has much higher stored curvature elastic stress than PI, supports the hypothesis that the activity of inositide phosphatase SopB has a physical role in vivo.

 Please wait while we load your content...
Please wait while we load your content...