Rhodium-catalyzed ortho-cyanation of symmetrical azobenzenes with N-cyano-N-phenyl-p-toluenesulfonamide†
Abstract
A rhodium-catalyzed ortho-cyanation of symmetrical azobenzenes is described employing N-cyano-N-phenyl-p-toluenesulfonamide as an environmentally friendly cyanide source. The present protocol allows the synthesis of various benzonitrile derivatives in moderate to good yields and tolerates many useful functional groups.
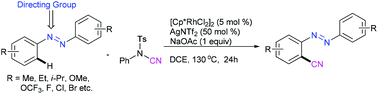
 Please wait while we load your content...
Please wait while we load your content...