A short designed semi-aromatic organic nanotube – synthesis, chiroptical characterization, and host properties†
Abstract
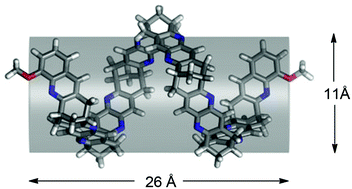
The first generation of an organic nanotube based on the enantiomerically pure bicyclo[3.3.1]nonane framework is presented. The helical tube synthesised is the longest to date having its aromatic systems oriented parallel to the axis of propagation (length ∼26 Å and inner diameter ∼11 Å according to molecular dynamics simulations in chloroform). The synthesis of the tube, a heptamer, is based on a series of Friedländer condensations and the use of pyrido[3,2-d]pyrimidine units as masked 2-amino aldehydes, as a general means to propagate organic tubular structures and the introduction of a methoxy group for modification toward solubility and functionalization are described. The electronic CD spectra of the tube and molecular intermediates are correlated with theoretical spectra calculated with time-dependent density functional theory to characterize the chirality of the tube. Both experimental (NMR-titrations) and theoretical (molecular dynamics simulations) techniques are used to investigate the use of the tube as a receptor for the acetylcholine and guanidinium cations, respectively.

 Please wait while we load your content...
Please wait while we load your content...