The stability of nitrogen-centered radicals†
Abstract
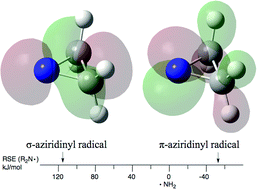
Radical stabilization energies (RSEs) for a wide variety of nitrogen-centered radicals and their protonated counterparts have been calculated at G3(MP2)-RAD and G3B3 level. The calculated RSE values can be rationalized through the combined effects of resonance delocalization of the unpaired spin, electron donation through adjacent alkyl groups or lone pairs, and through inductive electron donation/electron withdrawal. The influence of ring strain effects as well as the synergistic combination of individual substituent effects (captodatively stabilized N-radicals) have also been explored. In symmetric N-radicals the substituents may also affect the relative ordering of electronic states. In most cases the π-type radical (unpaired spin distribution perpendicular to the plane of the N-radical) is found to be most stable. Closed shell precursors of biological and pharmaceutical relevance, for which neither experimental nor theoretical results on radical stabilities exist, have been included.

 Please wait while we load your content...
Please wait while we load your content...