Enantioselective synthesis of 3-substituted 1,2-oxazinanes via organocatalytic intramolecular aza-Michael addition†
Abstract
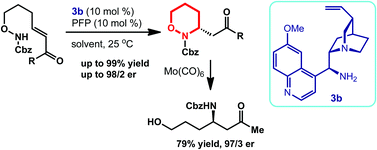
A highly enantioselective intramolecular 6-exo-trig aza-Michael addition was developed to afford chiral 3-substituted 1,2-oxazinanes in high yields (up to 99% yield) and good enantioselectivities (up to 98/2 er). These reactions were enabled by a quinine-derived primary–tertiary diamine as a catalyst and pentafluoropropionic acid (PFP) as a co-catalyst.
 Please wait while we load your content...
Please wait while we load your content...