An efficient continuous flow approach to furnish furan-based biaryls†
Abstract
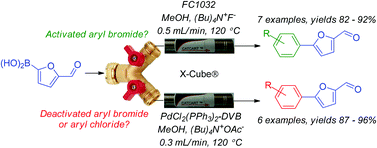
Suzuki cross-couplings of 5-formyl-2-furanylboronic acid with activated or neutral aryl bromides were performed under continuous flow conditions in the presence of (Bu)4N+F− and the immobilised t-butyl based palladium catalyst CatCart™ FC1032™. Deactivated aryl bromides and activated aryl chlorides were cross-coupled with 5-formyl-2-furanylboronic in the presence of (Bu)4N+OAc− using the bis-triphenylphosphine CatCart™ PdCl2(PPh3)2-DVB. Initial evidence indicates the latter method may serve as a universal approach to conduct Suzuki cross-couplings with the protocol successfully employed in the synthesis of the current gold standard Hedgehog pathway inhibitor LDE225.
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...