Oxidative damage of aromatic dipeptides by the environmental oxidants NO2˙ and O3†
Abstract
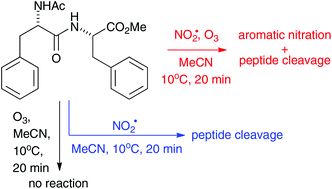
Irreversible oxidative damage at both aromatic side chains and dipeptide linkage occurs in the aromatic N- and C-protected dipeptides 7–11 upon exposure to the environmental pollutants NO2˙ and O3. The reaction proceeds through initial oxidation of the aromatic ring by in situ generated NO3˙, or by NO2˙, respectively, which leads to formation of nitroaromatic products. The indole ring in Phe-Trp undergoes oxidative cyclization to a pyrroloindoline. An important reaction pathway for dipeptides with less oxidisable aromatic side chains proceeds through fragmentation of the peptide bond with concomitant acyl migration. This process is likely initiated by an ionic reaction of the amide nitrogen with the NO2˙ dimer, N2O4.

 Please wait while we load your content...
Please wait while we load your content...