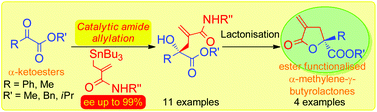
Catalytic amide allylation of α-ketoesters: extremely high enantioselective synthesis of ester functionalised α-methylene-γ-butyrolactones†
Abstract
This communication reports a significant breakthrough on the novel catalytic amide allylation to the acyclic α-ketoester systems, achieving satisfactorily high yields and extremely high levels of the asymmetric induction to allow for highly enantioselective synthesis of ester functionalised α-methylene-γ-butyrolactones.

 Please wait while we load your content...
Please wait while we load your content...