Total synthesis and biological evaluation of atrop-O-benzyl-desmethylabyssomicin C†
Abstract
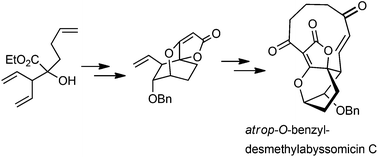
The total synthesis of desmethylabyssomicin C analogue 1 was accomplished using diastereotopos-selective ring closing metathesis and Nozaki–Hiyama–Kishi cyclization as the key steps. The synthetic analogue retained its antibacterial activity against methicillin-resistant S. aureus strains, whereas its cytotoxicity decreased for three orders of magnitude, as compared to atrop-abyssomicin C.

 Please wait while we load your content...
Please wait while we load your content...