1,3-Halogen migration as an entry to aryl coppers from an unintuitive starting material
Abstract
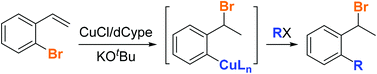
A copper(I) catalyzed 1,3-halogen migration/borylation migrates a bromine from an sp2 carbon to a benzylic carbon with concomitant borylation of the aryl–bromine bond. This transformation proceeds via an aryl copper intermediate which can be accessed independently and then trapped with electrophiles. As such, copper-catalyzed 1,3-halogen migration provides unique and mild access to an aryl copper species that allows for rapid aromatic functionalization from an unconventional starting material.

 Please wait while we load your content...
Please wait while we load your content...