Short asymmetric synthesis of phenanthroindolizidines through chiral homoallylic sulfinamines†
Abstract
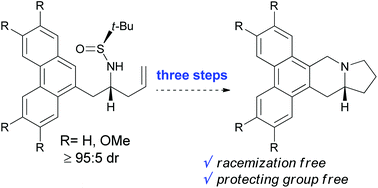
An efficient stereocontrolled preparation of chiral phenanthroindolizidines is detailed. The synthesis relies on the stereoselective indium-mediated allylation of 2-(phenanthren-9-yl)acetaldehyde derivatives with chiral tert-butylsulfinamide. Chemoselective transformations from the corresponding homoallylic sulfinamine allow the synthesis of the phenanthroindolizidines in only three synthetic operations, without any detectable racemization. Following this procedure, the synthesis of natural (−)-tylophorine was successfully accomplished.

 Please wait while we load your content...
Please wait while we load your content...