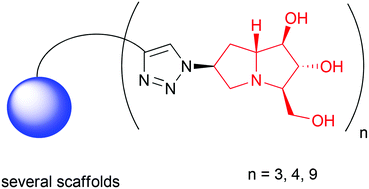
The synthesis of the first multivalent pyrrolizidine iminosugars is reported. The key azido intermediates 4 and 31 were prepared after suitable synthetic elaboration of the cycloadduct obtained from 1,3-dipolar cycloaddition of D-arabinose derived nitrone to dimethylacrylamide. The key step of the strategy was the stereoselective installation of an azido moiety at C-6 of the pyrrolizidine skeleton. The click reaction with different monovalent and dendrimeric alkyne scaffolds allowed the preparation of a library of new mono- and multivalent pyrrolizidine compounds that were preliminarily assayed as glycosidase inhibitors towards a panel of commercially available glycosyl hydrolases.