Expedient synthesis of an α-S-(1→6)-linked pentaglucosyl thiol†
Abstract
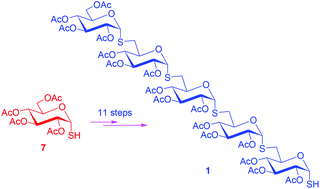
An α-S-(1→6)-linked pentaglucosyl thiol has been synthesized in a convenient and stereoselective way. Key steps of the synthesis involved thioglycosylation of 6-iodinated sugars with α-glycosyl thiols under phase transfer conditions. The α-configuration of glycosidic linkages was thus introduced prior to the coupling steps, and relied on the intrinsic configurational stability of α-glycosyl thiols. This work also demonstrated the great utility of MMTr as an effective anomeric S-protecting group.

 Please wait while we load your content...
Please wait while we load your content...