ent-Kaurane-based regio- and stereoselective inverse electron demand hetero-Diels–Alder reactions: synthesis of dihydropyran-fused diterpenoids†
Abstract
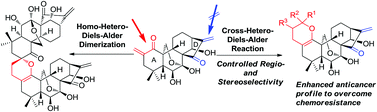
A mild and concise approach for the construction of a 3,4-dihydro-2H-pyran ring integrated into the A-ring of the natural product oridonin using an optimized inverse electron demand hetero-Diels–Alder (IED HDA) reaction is reported herein. A self-dimerization of the exocyclic enone installed in the A-ring through a homo-HDA reaction was identified to exclusively give a dimeric ent-kaurane diterpenoid with the spirochroman core. Moreover, efficient cross-HDA cycloadditions of this enone with various vinyl ethers or vinyl sulfides, instead of its own homo-HDA dimerization, were achieved in a regio- and stereoselective manner, thus providing access to novel dihydropyran-fused diterpenoids as potential anticancer agents to overcome chemoresistance.

 Please wait while we load your content...
Please wait while we load your content...