Abstract
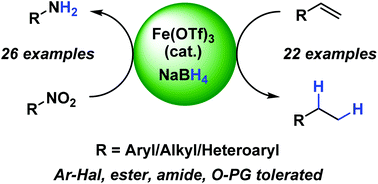
An operationally simple and environmentally benign formal hydrogenation protocol has been developed using highly abundant iron(III) salts and an inexpensive, bench stable, stoichiometric reductant, NaBH4, in ethanol, under ambient conditions. This reaction has been applied to the reduction of terminal alkenes (22 examples, up to 95% yield) and nitro-groups (26 examples, up to 95% yield). Deuterium labelling studies indicate that this reaction proceeds via an ionic rather than radical mechanism.
- This article is part of the themed collection: Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...