Base mediated 7-exo-dig intramolecular cyclization of Ugi–propargyl precursors: a highly efficient and regioselective synthetic approach toward diverse 1,4-benzoxazepine-5(2H)-ones†
Abstract
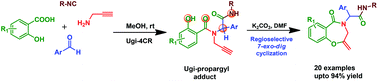
A metal-free facile and efficient two-step synthetic protocol for the preparation of 1,4-benzoxazepine-5(2H)-one derivatives has been developed. The protocol involves Ugi reaction followed by K2CO3 mediated highly regioselective 7-exo-dig intramolecular cyclization of less-nucleophilic oxygen with the pendant alkyne moiety of an Ugi–propargyl precursor to afford the 1,4-benzoxazepine-5(2H)-one derivatives in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...