Design, synthesis, conformational analysis and application of indolizidin-2-one dipeptide mimics
Abstract
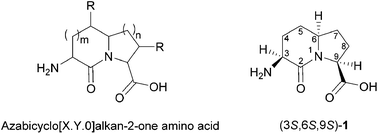
Growth in the field of peptide mimicry over the past few decades has resulted in the synthesis of many new compounds and the investigation of novel pharmacological agents. Azabicyclo[X.Y.0]alkanone amino acids are among the attractive classes of constrained mimics, because they can create rigid peptide structures for probing the conformation and roles of natural motifs in recognition events important for biological activity. Herein, we review the last ten years of the synthesis, conformational analysis and activity of analogs of the azabicyclo[4.3.0]alkan-2-one amino acid subclass, so-called indolizidin-2-one amino acids, with particular attention on their employment as inputs for biological applications.

 Please wait while we load your content...
Please wait while we load your content...