Hydrogen bond directed epoxidation: diastereoselective olefinic oxidation of allylic alcohols and amines†
Abstract
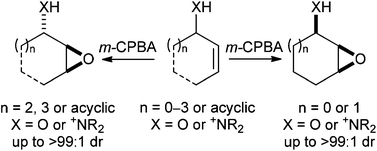
This article compares the diastereoselective epoxidation of acyclic and cyclic allylic alcohols, with the chemo- and diastereoselective olefinic oxidation of a range of acyclic and cyclic allylic amines. The diastereoselectivity in these systems is compared and a discussion about the origin of this high diastereocontrol is also presented. The ammonium directed epoxidation methodology has been extended to more complex substrates and representative applications of this protocol in natural product synthesis are also summarised.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday
 Please wait while we load your content...
Please wait while we load your content...