An ebselen like catalyst with enhanced GPx activity via a selenol intermediate†
Abstract
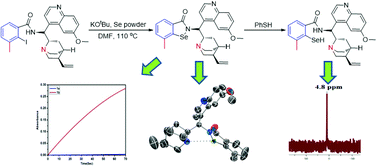
The reaction of KSeOtBu with 2-iodo-arylbenzamides gave benzamide ring-substituted, quinine-derived isoselenazolones 1b–1d. The reaction of PhSH with ortho-methyl-substituted isoselenazolone 1b gave selenol 3b, which is oxidized by H2O2 to regenerate 1b. Isoselenazolone 1b shows a high rate (0.33 × 103 μM min−1) of oxidation of PhSH with H2O2, which is ∼103-fold more active than ebselen (1a) and ≥30-fold more active than the other isoselenazolones of this study. Compound 1b shows less inhibition of the growth of yeast cells than 1a.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...