Non-stoichiometric O-acetylation of Shigella flexneri 2a O-specific polysaccharide: synthesis and antigenicity†
Abstract
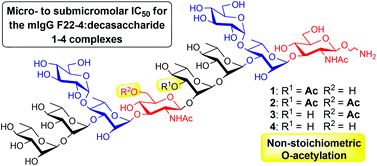
Synthetic functional mimics of the O-antigen from Shigella flexneri 2a are seen as promising vaccine components against endemic shigellosis. Herein, the influence of the polysaccharide non-stoichiometric di-O-acetylation on antigenicity is addressed for the first time. Three decasaccharides, representing relevant internal mono- and di-O-acetylation profiles of the O-antigen, were synthesized from a pivotal protected decasaccharide designed to tailor late stage site-selective O-acetylation. The latter was obtained via a convergent route involving the imidate glycosylation chemistry. Binding studies to five protective mIgGs showed that none of the acetates adds significantly to broad antibody recognition. Yet, one of the five antibodies had a unique pattern of binding. With IC50 in the micromolar to submicromolar range mIgG F22-4 exemplifies a remarkable tight binding antibody against diversely O-acetylated and non-O-acetylated fragments of a neutral polysaccharide of medical importance.

 Please wait while we load your content...
Please wait while we load your content...