Measurement of supramolecular effective molarities for intramolecular H-bonds in zinc porphyrin–imidazole complexes†
Abstract
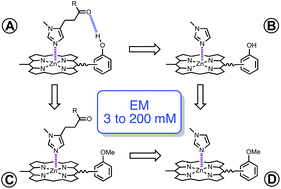
The association constants for formation of 1 : 1 complexes between five different imidazole ligands and eight different porphyrins have been measured by UV/vis titration experiments in two different solvents, toluene and 1,1,2,2-tetrachloroethane (TCE). Ligands equipped with H-bond acceptors (ester or amide) and porphyrins equipped with H-bond donors (phenol) can make H-bonds in addition to the zinc–nitrogen coordination interaction. The free energy contributions of these H-bonds to the overall stabilities of the complexes were determined using chemical double mutant cycles. Amide-phenol H-bonds contribute up to 5 kJ mol−1 to the free energy change on complexation, and ester-phenol H-bonds contribute up to 3 kJ mol−1. Porphyrin–ligand combinations with poor geometric complementarity do not make detectable H-bonding interactions. Effective molarities (EM) for the formation of H-bonds in the complexes were estimated by comparing the equilibrium constants for formation of the intramolecular interaction with the corresponding intermolecular interaction: the values are between 3 mM and 200 mM, which is comparable to previous results obtained for porphyrin–pyridine complexes. The values of EM measured for flexible and rigid ligand systems are comparable. This suggests that there is a trade off between restriction of conformational mobility in the flexible ligands and geometric strain in the rigid ligands, which results in similar binding affinities.

 Please wait while we load your content...
Please wait while we load your content...