Bromo- and thiomaleimides as a new class of thiol-mediated fluorescence ‘turn-on’ reagents†
Abstract
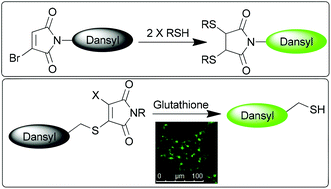
Bromo- and thiomaleimides are shown to serve as highly effective quenchers of a covalently attached fluorophore. Reactions with thiols that lead to removal of the maleimide conjugation, or detachment of the fluorophore from the maleimide, result in ‘turn-on’ of the fluorescence. These reagents thus offer opportunities in thiol sensing and intracellular reporting.

 Please wait while we load your content...
Please wait while we load your content...