Synthesis of substituted indenones and indanones by a Suzuki–Miyaura coupling/acid-promoted cyclisation sequence†
Abstract
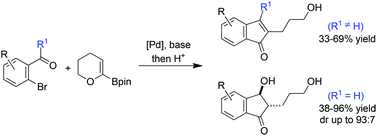
A one-pot Suzuki–Miyaura cross-coupling/acid-catalyzed cyclisation leading to indenones and indanones in modest to good yields is reported.

 Please wait while we load your content...
Please wait while we load your content...