Computational study of diastereoselective ortho-lithiations of chiral ferrocenes†
Abstract
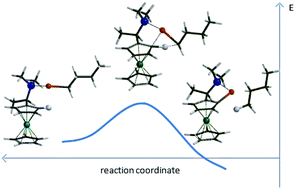
Diastereoselective ortho-lithiations serve for the preparation of many important chiral ferrocenes, however diastereoselections of these lithiations are explained only by simple steric models. We elucidated ortho-lithiations of three important ferrocenes based on DFT calculations. The calculations showed that simple models of transition states involving ferrocenyl substrates and lithium bases can only in some cases account for the experimental results. Transition state models, which include solvent or coordinating additives as distinct entities bound to lithium, can satisfactorily explain diastereoselection of the ortho-lithiations of chiral ferrocenes.

 Please wait while we load your content...
Please wait while we load your content...