Triangular Ag–Pd alloy nanoprisms: rational synthesis with high-efficiency for electrocatalytic oxygen reduction†
Abstract
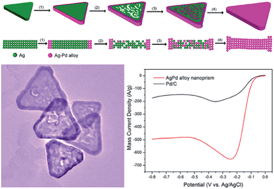
We report the generation of triangular Ag–Pd alloy nanoprisms through a rationally designed synthetic strategy based on silver nanoprisms as sacrificial templates. The galvanic replacement between Ag nanoprisms and H2PdCl4 along with co-reduction of Ag+/Pd2+ is responsible for the formation of final prismatic Ag–Pd alloy nanostructures. Significantly, these Ag–Pd alloy nanoprisms exhibited superior electrocatalytic activity for the oxygen reduction reaction (ORR) as compared with the commercial Pd/C catalyst. Such a high catalytic activity is attributed to not only the alloyed Ag–Pd composition but also the dominant {111} facets of the triangular Ag–Pd nanoprisms. This work demonstrates the rational design of bimetallic alloy nanostructures with control of selective crystal facets that are critical to achieve high catalytic activity for fuel cell systems.


 Please wait while we load your content...
Please wait while we load your content...