Photoluminescence, chemiluminescence and anodic electrochemiluminescence of hydrazide-modified graphene quantum dots†
Abstract
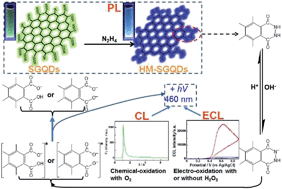
Single-layer graphene quantum dots (SGQDs) were refluxed with hydrazine (N2H4) to prepare hydrazide-modified SGQDs (HM-SGQDs). Compared with SGQDs, partial oxygen-containing groups have been removed from HM-SGQDs. At the same time, a lot of hydrazide groups have been introduced into HM-SGQDs. The introduced hydrazide groups provide HM-SGQDs with a new kind of surface state, and give HM-SGQDs unique photoluminescence (PL) properties such as blue-shifted PL emission and a relatively high PL quantum yield. More importantly, the hydrazide-modification made HM-SGQDs have abundant luminol-like units. Accordingly, HM-SGQDs exhibit unique and excellent chemiluminescence (CL) and anodic electrochemiluminescence (ECL). The hydrazide groups of HM-SGQDs can be chemically oxidized by the dissolved oxygen (O2) in alkaline solutions, producing a strong CL signal. The CL intensity is mainly dependent on the pH value and the concentration of O2, implying the potential applications of HM-SGQDs in pH and O2 sensors. The hydrazide groups of HM-SGQDs can also be electrochemically oxidized in alkaline solutions, producing a strong anodic ECL signal. The ECL intensity can be enhanced sensitively by hydrogen peroxide (H2O2). The enhanced ECL intensity is proportional to the concentration of H2O2 in a wide range of 3 μM to 500 μM. The detection limit of H2O2 was calculated to be about 0.7 μM. The results suggest the great potential applications of HM-SGQDs in the sensors of H2O2 and bio-molecules that are able to produce H2O2 in the presence of enzymes.

 Please wait while we load your content...
Please wait while we load your content...