Graphene oxide assisted spontaneous growth of V2O5 nanowires at room temperature†
Abstract
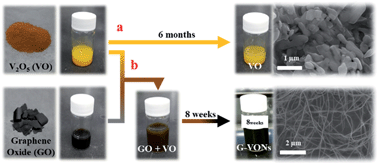
Graphene-decorated single crystalline V2O5 nanowires (G–VONs) have been synthesized by mixing graphene oxide (GO) and V2O5 suspensions at room temperature. In this process, V2O5 nanowires (VONs) are formed spontaneously from commercial V2O5 particles with the aid of GO. The as-formed one dimensional G–VONs were characterized by using a X-ray diffractometer, a X-ray photoelectron spectrometer, a scanning electron microscope, and a transmission electron microscope. GO plays a vital role in the VON formation with the simultaneous reduction of GO. A single G–VON showed superior electrical conductivity compared with that of the pure VONs obtained from the sol–gel method. This could be ascribed to the insertion of rGO sheets into the V2O5 layered structure, which was further confirmed by electron energy loss spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...