Polymorphic phase transition among the titania crystal structures using a solution-based approach: from precursor chemistry to nucleation process
Abstract
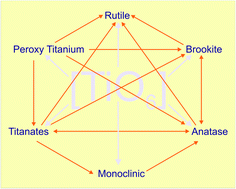
Nanocrystalline titania are a robust candidate for various functional applications owing to its non-toxicity, cheap availability, ease of preparation and exceptional photochemical as well as thermal stability. The uniqueness in each lattice structure of titania leads to multifaceted physico-chemical and opto-electronic properties, which yield different functionalities and thus influence their performances in various green energy applications. The high temperature treatment for crystallizing titania triggers inevitable particle growth and the destruction of delicate nanostructural features. Thus, the preparation of crystalline titania with tunable phase/particle size/morphology at low to moderate temperatures using a solution-based approach has paved the way for further exciting areas of research. In this focused review, titania synthesis from hydrothermal/solvothermal method, conventional sol–gel method and sol–gel-assisted method via ultrasonication, photoillumination and ILs, thermolysis and microemulsion routes are discussed. These wet chemical methods have broader visibility, since multiple reaction parameters, such as precursor chemistry, surfactants, chelating agents, solvents, mineralizer, pH of the solution, aging time, reaction temperature/time, inorganic electrolytes, can be easily manipulated to tune the final physical structure. This review sheds light on the stabilization/phase transformation pathways of titania polymorphs like anatase, rutile, brookite and TiO2(B) under a variety of reaction conditions. The driving force for crystallization arising from complex species in solution coupled with pH of the solution and ion species facilitating the orientation of octahedral resulting in a crystalline phase are reviewed in detail. In addition to titanium halide/alkoxide, the nucleation of titania from other precursors like peroxo and layered titanates are also discussed. The non-aqueous route and ball milling-induced titania transformation is briefly outlined; moreover, the lacunae in understanding the concepts and future prospects in this exciting field are suggested.

 Please wait while we load your content...
Please wait while we load your content...