Phase-segregated Pt–Ni chain-like nanohybrids with high electrocatalytic activity towards methanol oxidation reaction†
Abstract
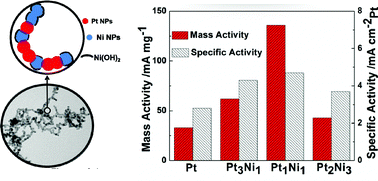
The phase-segregated Pt–Ni chain-like nanostructures, composed of monometallic counterparts attached to each other, were synthesized via a modified polyol process with the assistance of a small amount of PVP. The molar ratio between Pt and Ni was tuned by simply adjusting the feed ratio of the precursors. High-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS) results reveal that atomic diffusion occurred at the interface of the granular subunits. The negative shift of the Pt4f7/2 peak in the XPS spectra indicates the electron transfer from Ni to Pt atoms, while the strong peaks at around 855.7 eV suggest the surface oxidation of the Ni nanoparticles, which was further confirmed by the cyclic voltammetry (CV) measurement. The electrocatalytic activities of the methanol oxidation reaction (MOR) were found to be higher for the phase-segregated structures relative to those for pure Pt nanoparticles, and the activities followed the sequences of Pt1Ni1 > Pt3Ni1 ∼ Pt2Ni3 > pure Pt. We believe that the modified electronic structures and the existence of nickel hydroxide both contributed to the improved catalytic activities.

 Please wait while we load your content...
Please wait while we load your content...