The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties†
Abstract
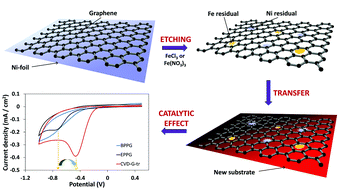
High quality graphene films can be fabricated by chemical vapor deposition (CVD) using Ni and Cu as catalytic substrates. Such a synthesis procedure always requires a subsequent transfer process to be performed in order to eliminate the metallic substrate and transfer the graphene onto the desired surface. We show here that such a transfer process causes significant contamination of the graphene film with residual Fe and Ni metal impurities. Fe contamination derives from the use of Fe-based etching solutions to dissolve Ni (or Cu) substrates, while residual Ni (or Cu) is due to an incomplete metal substrate etching. The presence of these metallic impurities within the transferred graphene film affects tremendously its electrochemical behavior when adopted as an electrode material.

 Please wait while we load your content...
Please wait while we load your content...