Selective catalytic burning of graphene by SiOx layer depletion†
Abstract
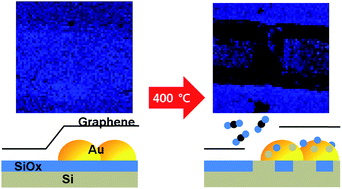
We report catalytic decomposition of few-layer graphene on an Au/SiOx/Si surface wherein oxygen is supplied by dissociation of the native SiOx layer at a relatively low temperature of 400 °C. The detailed chemical evolution of the graphene covered SiOx/Si surface with and without gold during the catalytic process is investigated using a spatially resolved photoelectron emission method. The oxygen atoms from the native SiOx layer activate the gold-mediated catalytic decomposition of the entire graphene layer, resulting in the formation of direct contact between the Au and the Si substrate. The notably low contact resistivity found in this system suggests that the catalytic depletion of a SiOx layer could realize a new way to micromanufacture high-quality electrical contact.

 Please wait while we load your content...
Please wait while we load your content...