Key role of ancillary ligands in imparting blue shift in electroluminescence wavelength in ruthenium polypyridyl light-emitting diodes†
Abstract
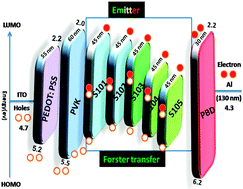
The synthesis, characterization and electrochemical, photoluminescence and electroluminescence properties of new ruthenium(II) polypyridyl complexes bearing dpq(COOH)2 ligand, [(phen/or bpy)nRu(L)3−n]2+ (n = 0, 1, 2), where bpy = 2,2-bipyridine, phen = 1,10-phenanthroline and L = 6,7-dicarboxylicdipyrido[2,2-d:2′,3′f] quinoxaline(dpq(COOH)2), coded as S101–S105, respectively, is presented. The five complexes differ in the number and type of ancillary groups, which are either bpy or phen. Cyclic voltammetry of S101–S105 exhibited a reversible one-electron oxidation wave and four one-electron reduction waves. The electroluminescence wavelengths of the complexes (S101–S105) were varied using different ancillary ligands from 485 to 572 nm, respectively. The role of ancillary ligands in the electroluminescence properties of Ru(dpq(COOH)2) complexes was investigated by comprehensive DFT and TD-DFT approaches which indicated that the lowest unoccupied molecular orbital (LUMO) of S101–S105 is localized in the distal portion of the dpq(COOH)2 ligand and the highest occupied molecular orbital (HOMO) in the donor region. The highest luminance of 1357 (cd m−2) and the lowest turn-on of 6.4 (V) were observed in light emitting diodes based on S101 containing 2 equivalents of bpy as the ancillary ligand and 1 equivalent of dpq(COOH)2, which is comparable to the maximum electroluminescence properties of Ru polypyridyl emitters. These values represent a new class in the field of organic light emitting diodes using less expensive Ru(II) polypyridyl complexes. The number of carboxylic moieties as substituents in these complexes played a significant role in lowering the tendency to aggregate and presenting a better electroluminescence efficiency than that of complexes derived from the phen ligand in a device. As an important result, the incorporation of bpy as an ancillary ligand to [Ru(dpq(COOH)2)] was found to be the most beneficial ancillary substitution in terms of decreasing the electroluminescence wavelength. These observations suggest that an ancillary ligand can be used to actively control the electroluminescence wavelength by means of tuning the energy level.

 Please wait while we load your content...
Please wait while we load your content...